Abstract
Activation of the JAK-STAT pathway is essential for the pathogenesis of primary myelofibrosis (PMF) and other myeloproliferative neoplasms (MPNs), but in the absence of curative treatment options, a major need remains to identify other targetable mechanisms in those diseases. We have previously shown that mice with conditional, inducible bone marrow-specific knockout of Abelson interactor 1 (Abi-1 KO) develop progressive myeloid hyperplasia, splenomegaly and bone marrow fibrosis leading to weight loss and decreased survival (Chorzalska, ASH 2016). Our data also indicated that hematopoietic progenitors from PMF patients show decreased levels of ABI1 transcript independent of the presence of JAK2 mutations. Abi-1 is one of several interactors of the Abelson kinase involved in Ras, PI3K and integrin signaling, and its deficiency in embryonic development leads to lethal defects of the heart and placenta. Despite suggestions that Abi-1 may act as a tumor suppressor, its involvement in hematopoiesis has not been elucidated.
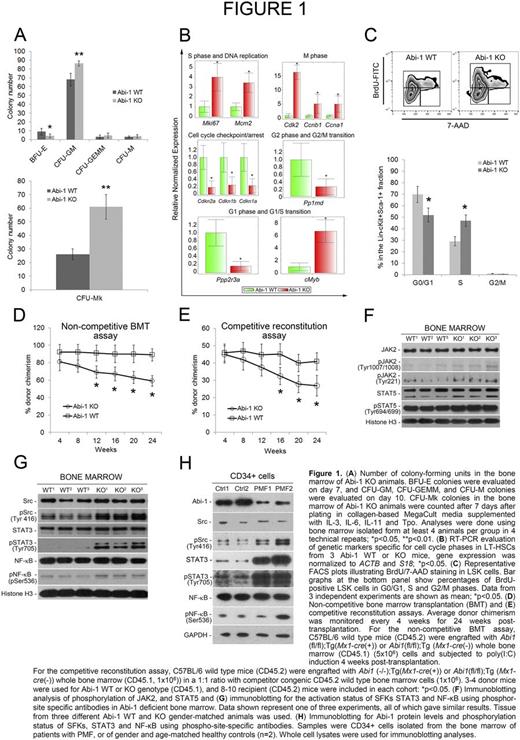
We have now characterized mechanisms that underlie the progressive PMF-like phenotype in our Abi-1 KO mice model. Colony-forming unit (CFU) assays indicated a significant decrease in the number of BFU-E colonies, a significant increase in CFU-GM colonies, and a nearly 3-fold increase in CFU-Mk colonies in the Abi-1 KO animals (Fig. 1A). Gene expression profiling studies showed a significant increase in the frequency of long-term hematopoietic stem cells (LT-HSCs) and HSCs enriched lineage- Sca-1+ cKit+ (LSK) cells in Abi-1 KO mice, associated with increased cell cycle activity in those cells: 3- to 4-fold higher expression of Mcm2 and Mki67 genes (specific to S phase and DNA replication), >6-fold upregulation of cMyb (specific for the G1/S phase transition), a 5-fold upregulation of M-phase specific Ccna1 and Ccnb1 and >15-fold upregulation of Cdk2, compared with wild-type (WT) animals. We also observed a 60 to 70% decrease in Cdkn1a, Cdkn1b, Cdkn2b (G1- or G2-phase-specific cell cycle inhibitors) transcripts in Abi-1 KO LT-HSCs (Fig. 1B). Investigation of cell cycle phases in HSC-enriched LSK cells using bromodeoxyuridine (BrdU) incorporation showed significantly more BrdU staining in cells from Abi-1 KO compared with WT mice, with a higher proportion of cells in S phase (Fig. 1C). Bone marrow transplantation assays done in C57BL/6 WT mice in the absence of competitor cells showed progressive loss of engraftment (decreased donor chimerism) of Abi-1 KO bone marrow cells over 6 months post transplant (Fig. 1D). Similarly, in competitive repopulation assays (C57BL/6 wild type mice engrafted with the whole bone marrow from Abi-1 KO or WT mice in 1:1 ratio with competitor, congenic CD45.2 WT cells), we observed a progressive decrease in donor chimerism with Abi1 inactivation (Fig. 1E).
Using the Abi-1 KO model we then evaluated signaling pathways critical for the MPN pathophysiology, detecting only a modest increase in JAK2 and STAT5 activities (Fig. 1F). Immunoblotting analyses of the activities of Src Family Kinases (SFKs) showed increased phosphorylation on Tyr 416, consistent with activation of SFKs as well as increased activity of STAT3 and NF-κB (Fig. 1G). We confirmed these findings in CD34+ cell isolates from PMF patients (n=2), showing a decrease in Abi-1 protein levels and significant upregulation of SFKs, STAT3 and NF-κB activities (Fig. 1H).
In summary, bone marrow-specific inactivation of Abi1 appears to be sufficient to induce a MPN-like phenotype, replicating features of human PMF. Results from gene expression profiling, immunoblotting, and proteomics consistently show that activation of SFKs, STAT3 and NF-κB may be involved in the genesis of this phenotype. We continue to explore the mechanisms responsible for the downregulation of ABI1 in hematopoietic progenitor cells from patients with PMF/MPN, as well as use of murine models with mutations in JAK2, CALR or MPL to determine mechanisms responsible for ABI1 loss and its association with classical MPN-associated pathways. Our work provides further evidence supporting a role for Abi-1 in pathogenesis of MPNs and a possible link to the SFK-STAT3-NF-κB signaling axis. Full elucidation of these mechanisms may couple the proliferative and pro-inflammatory phenomena that characterize PMF/MPNs, and provide a new therapeutic strategy for these diseases.
Reagan: Teva: Membership on an entity's Board of Directors or advisory committees. Vannucchi: Shire: Speakers Bureau; Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal